Sinovac Vaccine Accepted In Usa
There are other vaccines that have been approved around the world. Second in line is Pfizer-BioNTech followed by Sputnik V.

Sinovac Covid 19 Vaccine Gets Approval In Thailand For Emergency Use
It announced that any Covid-19 vaccine approved for emergency use by the World Health Organization is accepted.
Sinovac vaccine accepted in usa. If the second dose is administered less than 2 weeks after the first the dose does not need to be repeated. It is recommended that all vaccinated individuals receive two doses. What vaccinations are likely to be accepted.
The world desperately needs multiple COVID-19 vaccines to. According to a new tool developed by VisaGuideWorld which helps travellers to find out whether the country they plan on visiting accepts their COVID-19 vaccine as valid proof it has been revealed that the three vaccines that are most recognised in EU countries after the EMA ones are Covishield Sinopharm and Sinovac. Otherwise they will be denied entry into Canada.
In addition to vaccines by Pfizer-BioNTech Moderna Inc AstraZeneca and Johnson Johnson WHO has given the green light to the two Chinese vaccines made by Sinovac and Sinopharm. MONTREAL - Although the AstraZeneca vaccine is not licensed for use in the United States US. 3 or so Johnson Johnsons is a one-shot vaccine so.
In the case of the PfizerBioNTech and Moderna vaccines it is widely known that their effectiveness rate are 95 percent and 94 percent respectively. At this time foreign national travellers who havent received the full series of a vaccine or a combination of vaccines accepted by the Government of Canada at least 14 days prior to entry cannot come to Canada unless they meet an exemption set out in the Orders made under the Quarantine Act. Varies widely one report suggests the range is from 5 ito 38 in the Philippines.
WHO today validated the Sinovac-CoronaVac COVID-19 vaccine for emergency use giving countries funders procuring agencies and communities the assurance that it meets international standards for safety efficacy and manufacturing. Vaccines made in third countries such as Russia and China which have not been approved by the EMA are accepted. Questions about Sinovac.
5th UPDATE Some countries may not accept travelers inoculated with the Sinovac COVID-19 vaccine which comprises the bulk of the Philippines. The vaccine is produced by the Beijing-based pharmaceutical company Sinovac. WHO has recommended the Sinovac-CoronaVac vaccine for use in adults 18 years and older in a two-dose schedule with a spacing of two to four weeks.
WHO recommends an interval of 24 weeks between the first and second dose. The Department of Health has asked the Foreign Affairs to confirm reports that some countries ban travelers vaccinated with Sinovac or Sinopharm COVID-19 vaccine. First why is it that in all the statements and reports on the Sinovac vaccine there isnt any mention of the vaccines effectivity rate.
CanSino and Sinovac vaccine forms may not be valid for crossing into the US. Coming in third and fourth places are Sinopharm and Moderna respectively. The vaccine must protect against a disease that has been eliminated or is in the process of being eliminated in the United States.
Denmark and Norway suspended the use of the OxfordAstraZeneca vaccine due to a small number of reports of a rare blood clot disorder. The Embassy issued the response after reporters sought comment on information circulating that travelers inoculated with China-made Sinovac vaccines have allegedly been denied entry to the US. The Sinovac vaccine contains proteins capable of stimulating the immune system to produce antibodies to fight COVID-19.
The vaccine must protect against a disease that has the potential to cause an outbreak. However only residents of Europe the United States the United. If you have received a first dose of any of these EUL listed vaccines you may follow up with a second dose of PfizerBioNTech vaccine from USC Student Health.
Vaccine efficacy results showed that the vaccine prevented. The above is due to the fact that the United States and China have trade problems derived from the Trump administration in 2018 when it. But also the AstraZeneca and Chinas Sinovac.
According to social media posts that quoted the General Authority of Civil Aviation GACA of Saudi Arabia travelers who received the said Chinese COVID-19 vaccines would be denied entry. The AstraZeneca vaccine is currently the most widely used and accepted across the world when it comes to travel with 119 governments recognising it according to research by The Economist. ACIP recommends vaccines for a.
The following WHO-approved emergency use listing EUL vaccines are accepted for meeting COVID-19 vaccine requirements. The United States Food and Drug Administration USFDA has approved Pfizer-BioNTech Moderna and Janssen coronavirus disease 2019 COVID-19 vaccines for emergency use. SAGE recommends the use of Sinovac-CoronaVac vaccine as 2 doses 05 ml given intramuscularly.
To use the Covid Vaccine Checker for Travelling Abroad select the vaccine you have taken and then select your destination country. The WHO is still reviewing Russias Sputnik V vaccine but hasnt approved it. Health authorities now officially consider people.
The vaccines can also be approved more swiftly on an emergent basis and released sooner for usage. Now for my questions. This includes the US-manufactured Pfizer Moderna and Johnson Johnson that are used in the United States.
The checker will then tell you whether your vaccine is accepted as valid for immunization against COVID-19 in your destination country or not. The United States does not require vaccination against COVID-19 as a condition of entry US Embassy Press Attaché Heather Fabrikant said. The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations.

Sinovac Covid 19 Vaccine Gets Emergency Use Approval In China
Who Approves Sinovac Covid Vaccine The Second Chinese Made Dose Listed

Who Approves China S Sinovac Vaccine For Emergency Use
Philippines Approves Sinovac Vaccine But Not For All Health Workers Reuters

Sinovac To Apply For Clinical Trial Of Delta Specific Vaccine In Q3 General Manager Global Times
Vaccine Checker Which Vaccine Is Approved For International Travel

U S Pushed Brazil To Reject Russia S Sputnik V Vaccine Hhs Report Says The Washington Post
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex

Eu Regulator Examines Sinovac S Covid 19 Vaccine

Sinovac Covid 19 Vaccine Granted Approval In China
China To Only Allow Foreign Visitors Who Have Had Chinese Made Vaccine China The Guardian
Sinopharm Sinovac Covid 19 Vaccine Data Show Efficacy Who World News Us News

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 16 09 2021

Who Grants Emergency Authorisation To Sinovac Vaccine Latest Updates
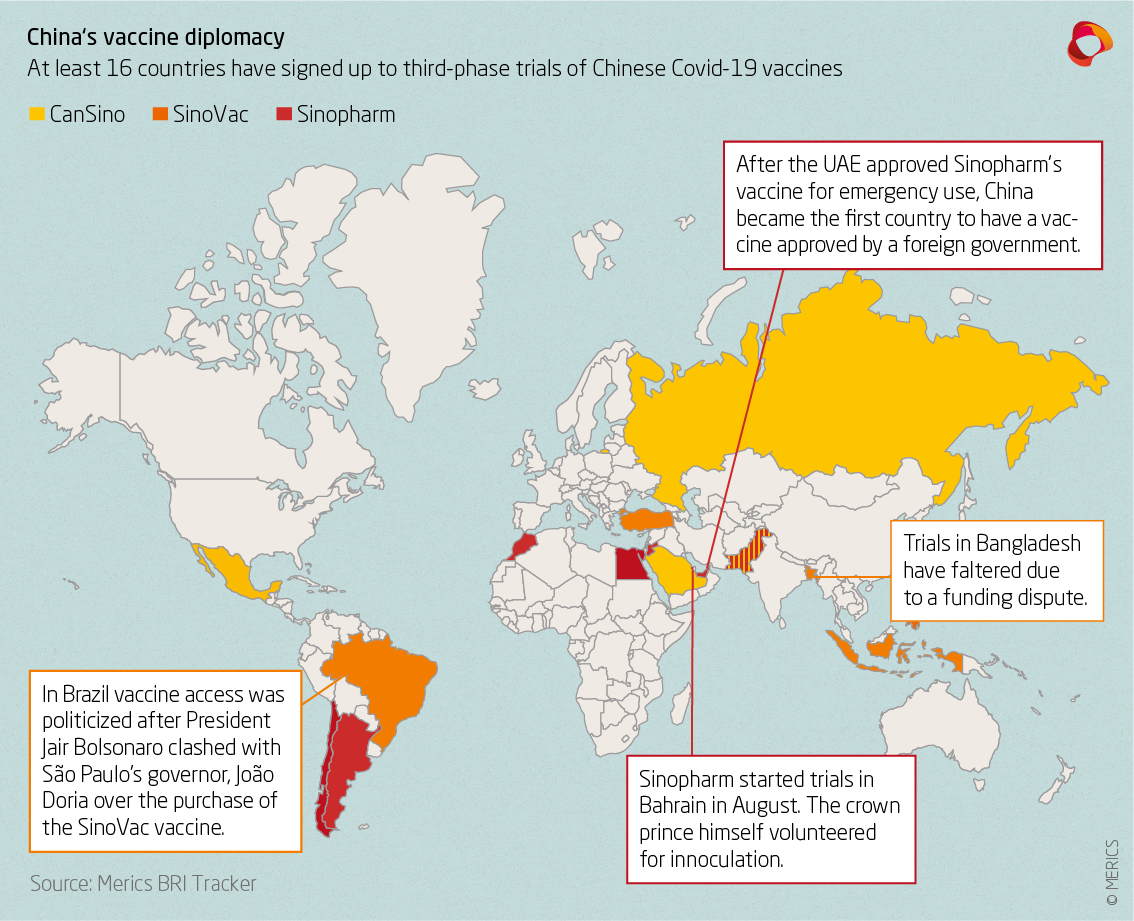
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Sinovac Vaccine Among Those Approved For Entry Into The U S

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 16 09 2021

Update 50 Countries Now Recognise Chinese Sinovac Vaccine For Travel Amid Covid 19 Visaguide World
Gavi Signs Agreements With Sinopharm And Sinovac For Immediate Supply To Covax Gavi The Vaccine Alliance

Posting Komentar untuk "Sinovac Vaccine Accepted In Usa"